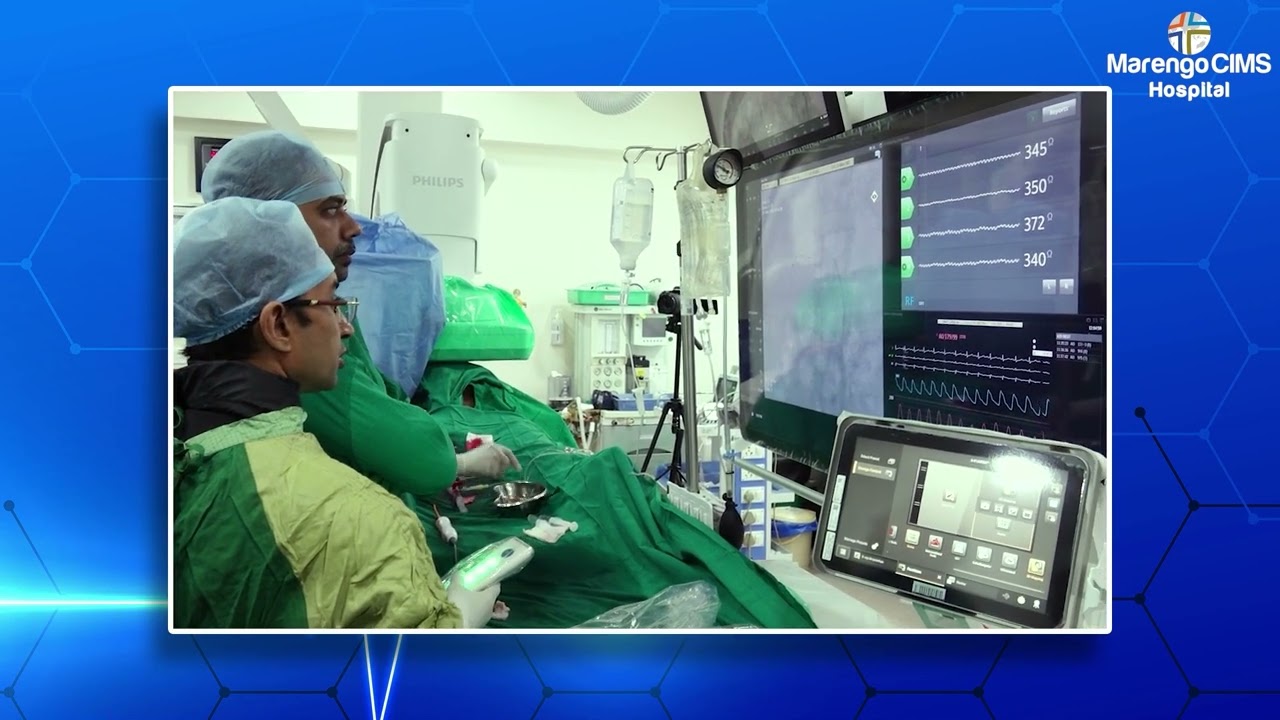
India has drawn nationwide media attention with the successful completion of its first Grassroot clinical trial of an indigenously developed brain stroke device, marking a major milestone in neurovascular care. The study highlights India’s growing strength in developing and clinically validating advanced medical technologies within the country.
The trial evaluated a Made-in-India neurovascular stent designed by Indian engineers and tested across eight reputed hospitals, including Marengo CIMS Hospital. Developed to address Indian anatomical and clinical needs, the device demonstrated safety and efficacy comparable to imported technologies while offering improved affordability and accessibility for acute ischemic stroke treatment.
Conducted under the leadership of Dr. Mukesh Sharma, Principal Investigator, the multicentric study assessed technical success, restoration of cerebral blood flow, safety outcomes, and patient recovery. The encouraging results have reinforced confidence in indigenous innovation for complex neuro-interventional procedures.
Aligned with the “Make in India” vision, this landmark trial is expected to reduce dependence on imported devices, lower treatment costs, and expand access to timely stroke care. Marengo CIMS Hospital’s participation reflects its continued commitment to research-driven, evidence-based patient care and national healthcare advancement.
 Like
0
Like
0
 Dislike
0
Dislike
0
 Love
0
Love
0
 Funny
0
Funny
0
 Angry
0
Angry
0
 Sad
0
Sad
0
 Wow
0
Wow
0