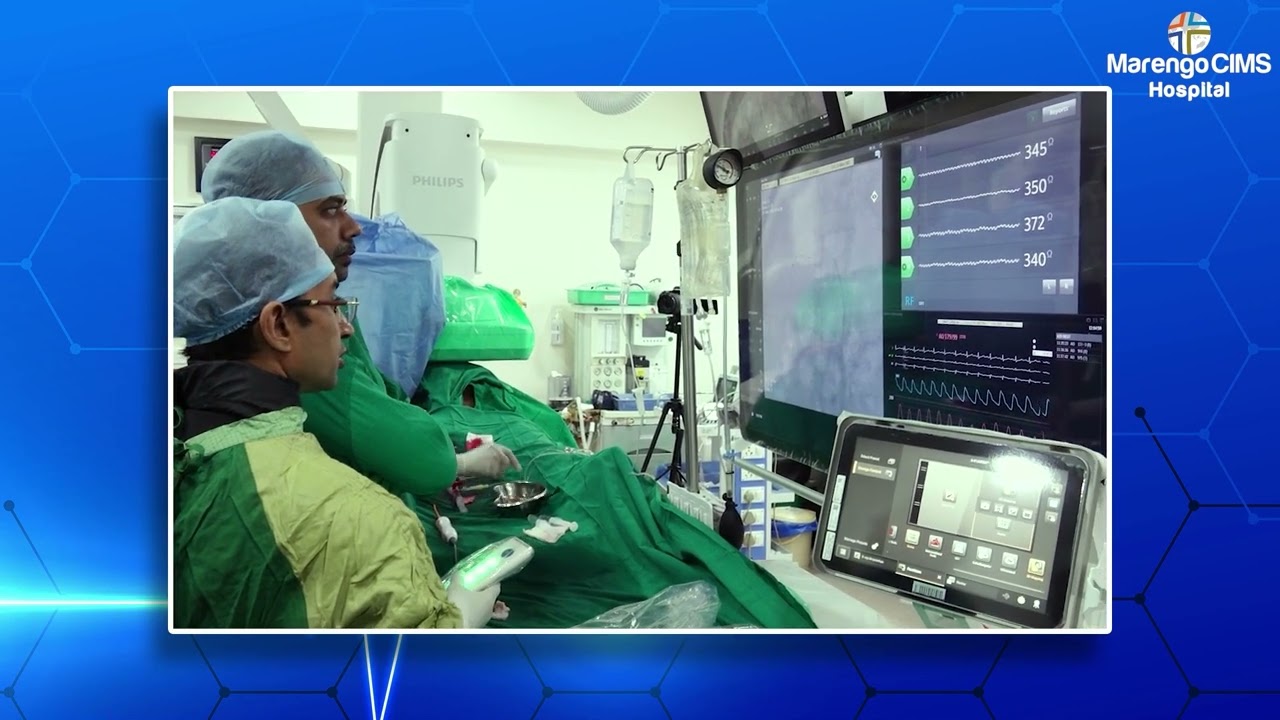
India has taken a significant step forward in advancing stroke care with the successful completion of the country’s first Grassroot clinical trial of an indigenously designed neurovascular stent. This landmark study represents a major leap in delivering high-quality, accessible stroke treatment within India. Designed by Indian engineers and tested across reputed tertiary-care centers, including Marengo CIMS Hospital, the trial highlights the growing strength of indigenous innovation in critical medical technologies.
The successful conduct of this trial marks an important milestone in India’s journey toward self-reliance in advanced healthcare solutions. In the context of acute ischemic stroke, where timely intervention can determine survival and long-term neurological outcomes, the availability of a safe, effective, and locally developed neurovascular device is a transformative development for both clinicians and patients.
Acute ischemic stroke occurs when a blood clot obstructs a major artery supplying the brain, leading to rapid loss of brain function. Mechanical thrombectomy has emerged as the gold-standard treatment for such cases; however, the widespread use of this life-saving procedure has been limited by the high cost of imported neurovascular devices. The newly developed Made-in-India brain vessel stent has been engineered specifically to address Indian anatomical needs and healthcare realities, offering comparable safety and efficacy while significantly improving affordability and accessibility.
The Grassroot trial was conducted across eight reputed hospitals in India, involving a multidisciplinary team of neurologists, interventional neuroradiologists, and stroke specialists. Marengo CIMS Hospital played a pivotal role as one of the key participating centers, actively contributing to patient enrolment, procedural implementation, and outcome evaluation. This multicentric approach ensured that the study reflected real-world clinical scenarios and diverse patient populations.
The research focused on critical clinical parameters, including the technical success of clot retrieval, restoration of cerebral blood flow, safety outcomes and complication rates, and functional recovery following stroke. The trial results demonstrated very high success rates with encouraging patient outcomes, reinforcing the effectiveness of indigenous innovation in complex neuro-interventional procedures.
The study was conducted at Marengo CIMS Hospital under the leadership of Dr. Mukesh Sharma, Principal Investigator, along with a dedicated team of stroke experts. This collaboration exemplifies the strength of Indian clinical research when supported by native engineering excellence and a shared commitment to innovation-driven healthcare solutions.
This indigenous neurovascular device strongly aligns with the “Make in India” vision by reducing dependence on imported medical technologies and enhancing the availability of advanced stroke care. The development promises to lower procedural costs, expand access to timely treatment, and support technologies tailored to Indian healthcare settings. Additionally, it strengthens India’s position as an emerging leader in global medical device innovation.
Marengo CIMS Hospital continues to demonstrate its commitment to research-driven, evidence-based patient care through its active participation in such landmark national trials. By contributing to clinical research and the adoption of cutting-edge therapies, the hospital reinforces its mission to deliver world-class healthcare grounded in strong scientific evidence and innovation.
 Like
0
Like
0
 Dislike
0
Dislike
0
 Love
0
Love
0
 Funny
0
Funny
0
 Angry
0
Angry
0
 Sad
0
Sad
0
 Wow
0
Wow
0